JBS Beads-for-Seeds-MiTeGen 制造微播种晶体种子
上海金畔生物代理MiTeGen品牌蛋白结晶试剂耗材工具等,我们将竭诚为您服务,欢迎访问MiTeGen官网或者咨询我们获取更多相关MiTeGen品牌产品信息。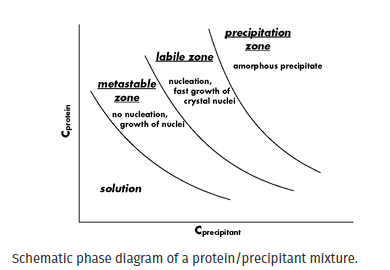
JBS Beads-for-Seeds
Preparation of seed stocks from protein crystals for microseeding applications. A highly polished glass bead and a microcentrifuge tube are used as mortar and pestle for crushing of seed crystals.
Content:
24 glass beads, each in a 1.5 ml microcentrifuge tube.
JBS Beads-for-Seeds
从蛋白质晶体中制备用于微播种应用的种子储备。 高度抛光的玻璃珠和微量离心管用作研钵和研杵,用于粉碎晶种。
内容:
24 个玻璃珠,每个在 1.5 ml 微量离心管中。
Full Description
Product Information
- Product Description
- Instructions
- Datasheet
Instructions for preparation of a seed stock
- Preparation of a stabilizing solution
A stabilizing solution should maintain the stability of a crystal, i.e. the crystal should not dissolve nor grow any further. Ideally, it should have the same composition as the solution of the drop from which the crystal was removed. This can be experimentally achieved by mixing the original sample solution with the drop reservoir. - Pipet 50 μl of stabilizing solution to the microcentrifuge tube containing the glass bead.
- Harvest a crystal from the drop using a MicroMount or MicroMesh inserted into a 0.7 mm mechanical pencil for better control. Remove excess liquid with a paper wick to minimize the carryover of liquid.
- Place the crystal in the microcentrifuge tube containing the glass bead and the stabilizing solution. Close the tube tightly.
- Vortex at medium speed for ca. 2 min. The glass bead should randomly bounce in the tube to ensure effective crushing.
- Add 450 μl stabilizing solution and mix thoroughly.
- Serial dilutions of the seed stock can be prepared by 1:10 dilutions of seed stock with stabilizing solution. The seed stocks may be successively diluted up to 10-5 times of its original concentration.
Please note: If your stabilization solution contains detergents or other additives which may foam vortexing is not recommended. Alternatively, the sample is treated exactly as described above, but instead of vortexing it is placed in an ultrasonic cleaner for two 1 min intervals.
Use a new microcentrifuge tube and a clean glass bead each time you prepare a new seed stock to avoid contamination and carryover from past experiments.
Performing the crystallization experiment
Prepare the crystallization drop by mixing protein sample and seed stock. Do not add reservoir solution since this may dissolve the seeds. Place the drops over a reservoir solution which has the same composition as the reservoir that was used to grow the initial seed crystal.
Ideally, the seeds should be transferred to a protein solution in the metastable zone, i.e. the solution should be slightly supersaturated.
Transferring seeds to an under saturated solution would result in the dissolution of the nuclei and no crystal growth would be observed. Transferring seeds to a highly supersaturated solution can yield showers of microcrystals.
产品信息
产品描述
指示
数据表
接种实验可以提高蛋白质结晶成功的可能性并优化生长条件。为了使生物大分子结晶,其浓度缓慢增加直至达到过饱和点。使用相图,可以在三个连续区域中显示过饱和度,即亚稳态过饱和度、不稳定过饱和度和沉淀(图 1)。在亚稳态区域,不会发生自发成核,但添加到该区域的晶体可以生长。在不稳定区,发生自发成核并观察到核的快速生长。在沉淀区,生物大分子多次过饱和,导致形成无定形沉淀。成核发生在比晶体生长更高的过饱和水平。通过将种子放入亚稳区过饱和的溶液中,可以优化生长条件并获得大的单晶。晶体生长的数量会受到添加到蛋白质滴中的种子储备浓度的影响。用非常浓缩的种子原料播种可能会导致微晶的淋漓。如果种子储备太稀,则没有细胞核会转移到蛋白质滴中。种子溶液的理想浓度可以通过从浓缩的种子库中进行连续稀释来通过实验确定。
准备种子股票的说明稳定溶液的制备
稳定溶液应保持晶体的稳定性,即晶体不应溶解或进一步生长。理想情况下,它应该具有与去除晶体的液滴溶液相同的成分。这可以通过将原始样品溶液与液滴储存器混合来通过实验实现。
吸取 50 μl 稳定溶液到含有玻璃珠的微量离心管中。
使用插入 0.7 毫米自动铅笔的 MicroMount 或 MicroMesh 从液滴中收集晶体,以便更好地控制。用纸芯去除多余的液体,以尽量减少液体的残留。
将晶体放入含有玻璃珠和稳定溶液的微量离心管中。紧紧关闭管子。
以中速涡旋约。 2 分钟。玻璃珠应在管内随机弹跳,以确保有效粉碎。
加入 450 μl 稳定液并调匀。
种子储备的连续稀释可以通过用稳定溶液按 1:10 稀释种子储备来制备。种子储备可连续稀释至其原始浓度的 10-5 倍。
请注意:如果您的稳定溶液含有清洁剂或其他可能产生泡沫的添加剂,则不推荐涡旋。或者,完全按照上述方法处理样品,但不是涡旋,而是将其放置在超声波清洁器中,间隔为两个 1 分钟。每次准备新的种子储备时,请使用新的微量离心管和干净的玻璃珠,以避免污染和过去实验的残留。
进行结晶实验
通过混合蛋白质样品和种子股票准备结晶下降。不要添加水库溶液,因为这可能会溶解种子。将液滴放在与用于生长初始晶种的储层具有相同成分的储层溶液上。
理想情况下,种子应转移到亚稳区的蛋白质溶液中,即溶液应略微过饱和。
将晶种转移到饱和溶液中会导致晶核溶解,并且不会观察到晶体生长。将种子转移到高度过饱和的溶液中可以产生微晶阵雨。
种子数据表珠
